1K
For our recent Researcher of the Month, we spoke to Professor Steve Winder, Professor of Molecular Cell Biology, at the Department of Biomedical Science, The University of Sheffield. His laboratory focuses on the study of dystroglycan, a protein that plays an important role in cell adhesion and signalling. His recent paper in Human Molecular Genetics speaks about the a FDA approved drug, currently being used for treating leukemia as a treatment for Duchenne Muscular Dystrophy (DMD). Here is Professor Win
der telling us more about his lab’s findings and how we might cure DMD in the near future.
CTS : For the benefit of our readers, could you please tell us more about your findings in the recent study.
SW: Identification of a systemically acting and universal small molecule therapy for Duchenne muscular dystrophy would be an enormous advance for this condition. Based on evidence gained from studies on mouse genetic models, we have identified tyrosine phosphorylation and degradation of β-dystroglycan as a key event in the aetiology of Duchenne muscular dystrophy. Thus, preventing tyrosine phosphorylation and degradation of β-dystroglycan presents itself as a potential therapeutic strategy.
SW: Identification of a systemically acting and universal small molecule therapy for Duchenne muscular dystrophy would be an enormous advance for this condition. Based on evidence gained from studies on mouse genetic models, we have identified tyrosine phosphorylation and degradation of β-dystroglycan as a key event in the aetiology of Duchenne muscular dystrophy. Thus, preventing tyrosine phosphorylation and degradation of β-dystroglycan presents itself as a potential therapeutic strategy.
Using the dystrophic sapje zebrafish, we have investigated the use of tyrosine kinase and other inhibitors to treat the dystrophic symptoms in this model of Duchenne muscular dystrophy.
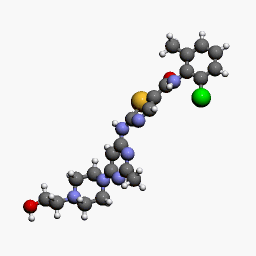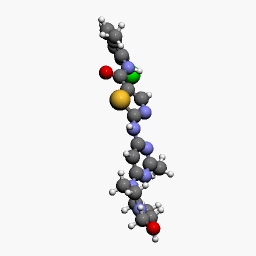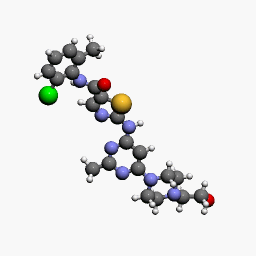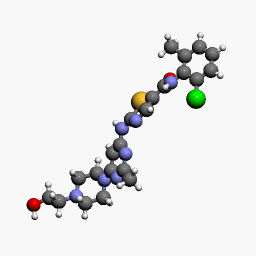 |
| Molecular Structure of Dasatinib, a drug produced by Bristol-Myers Squibb and marketed as Sprycel |
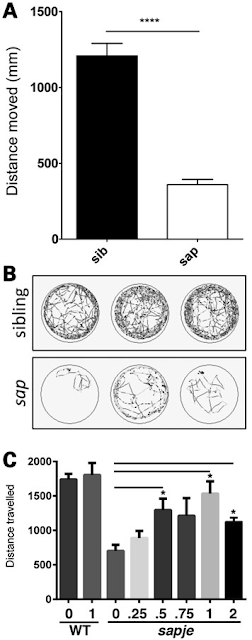
Dasatinib, a potent and specific Src tyrosine kinase inhibitor, was found to decrease the levels of β-dystroglycan phosphorylation on tyrosine and to increase the relative levels of non-phosphorylated β-dystroglycan in sapje zebrafish. Furthermore, dasatinib treatment resulted in the improved physical appearance of the sapje zebrafish musculature and increased swimming ability as measured by both duration and distance of swimming of dasatinib-treated fish compared with control animals. These data suggest great promise for pharmacological agents that prevent the phosphorylation of β-dystroglycan on tyrosine and subsequent steps in the degradation pathway as therapeutic targets for the treatment of Duchenne muscular dystrophy.
CTS: Of all Src inhibitors available, Why was dastanib chosen?
SW:We actually used the zebrafish system to screen many compounds and drugs. Dasatinib was just picked as an exemplar for publication.
CTS: Korner et al 2014 carried out a similar study with a compound called bortezomib. How different would you say is this study from theirs?
SW: Korner et al used a mouse model of congenital muscular dystrophy(CMD) and treated it with a proteasome inhibitor, whereas we used a mouse model of DMD and a Src inhibitor. Interestingly the same group have recently shown that the same drug is not effective against a different mousemodel of the same disease.
CTS: So, would you be working on a different model of zebrafish to ensure that that your findings are reproducible?
SW: Given that we have already advanced our studies in mdx mice with some apparent success, there seems little need to go back to zebrafish at this stage.
CTS: Your lab has worked on mouse models earlier, why shift to zebrafish for this study?
SW: The mouse model demonstrated the importance of tyrosine phosphorylation in the aetiology of DMD. The shift to fish was simply for the purposes of screening many candidate compounds. Drug screening is much quicker, cheaper and easier in fish than in mice.
CTS:What happens next? Since this is an approved drug, can we skip the human trials altogether?
SW:No, trials are still needed. Although dasatinib is approved clinically and the safety testing has been done, we will still need to demonstrate efficacy against DMD in people.
CTS: So, would your lab be involved in the clinical trials for Duchenne Muscular Dystrophy?
SW: The initial discovery is patented with us as inventors, however pharma involvement would be needed in order to obtain the drugs for the trial. The trial could be investigator led, or pharma could choose to do it.
 |
| Just a regular day at the office. Image credit: Professor Steve Winder |
CTS: Looking at the treatments in the future, isn’t gene therapy a more robust answer to a genetic disease like DMD. When perfected, it holds promise of 100% recovery. Is your lab looking at gene replacement therapies as well?
SW: Whilst DMD is a monogenic disease, there are hundreds of different mutations. There is currently no single genetic therapy that is capable of correcting all the defects, and those that there are, are far from 100% perfect. Delivering these sorts of therapies to muscle remains problematic. CRISPR-Cas9 mediated gene editing would appear to be one way around that.
However, we are not pursuing gene therapy approaches since that is not our current area of expertise.For the moment, a systemic small molecule inhibitor could be an answer. So although at present we are still working up the pharmacological approach, but ultimately a combinatorial regimen of pharmacological and genetic therapy may give greatest efficacy.
Readers interested to know about Duchenne Muscular Dystrophy would like to read our other post, Exception to the thumb rule.
If you would like to read more of these interesting stories from the world of science, subscribe to our blog and we will send you an email every time we post something new and interesting. Alternatively, you can follow us on social media such as Facebook, Twitter or Google Plus!
References:
If you would like to read more of these interesting stories from the world of science, subscribe to our blog and we will send you an email every time we post something new and interesting. Alternatively, you can follow us on social media such as Facebook, Twitter or Google Plus!
References:
Körner Z, Fontes-Oliveira CC, Holmberg J, Carmignac V, & Durbeej M (2014). Bortezomib partially improves laminin α2 chain-deficient muscular dystrophy. The American journal of pathology, 184 (5), 1518-28 PMID: 24631023
Körner Z, & Durbeej M (2016). Bortezomib Does Not Reduce Muscular Dystrophy in the dy2J/dy2J Mouse Model of Laminin α2 Chain-Deficient Muscular Dystrophy. PloS one, 11 (1) PMID: 26731667
Lipscomb L, Piggott RW, Emmerson T, & Winder SJ (2016). Dasatinib as a treatment for Duchenne muscular dystrophy. Human molecular genetics, 25 (2), 266-74 PMID: 26604135