Like most kids growing up in India, I was encouraged to take up science after my secondary school and expected to make a career in medicine or engineering somewhere. While the debate will always continue whether such encouragement is good for the child or not, as a student of science, there was a lot of information that was being bombarded at me, some of which I liked and some of which I just could not get my head around. Unfortunately, Chemistry features in the list of subjects, I didn’t understand completely.
We studied chemistry to know how the end product is made.
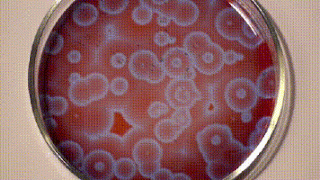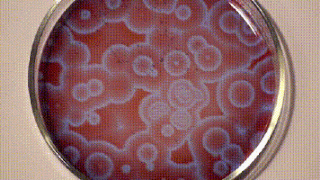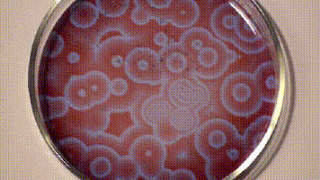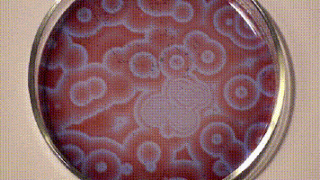
It was only a decade later that Anatol Zhabotinsky, who was working at the University of Moscow came across Belousov’s findings and investigated them further. Unfortunately, he, too, wasn’t able to publish his findings at that time and it was only another decade later in 1972 when the mechanism of oscillating reactions was finally published. The reason for colour changes of the solution is the oscillating oxidation state of Cerium which is yellow in a higher oxidation state and colourless in a lower oxidation state.
Since then, there are many reaction mixes that have been described that follow the principle of Belousov-Zhabotinsky reaction and one such is shown below using ferroin, which I found on Stephen Morris’ YouTube Channel.
I am quite sure that had some one showed me this video 10 years ago, my attitude towards chemistry would have been very very different. Would yours have been?
Do let us me know using the comments section below!