1K
 |
| Marta Llimargas (Right) with co-authors for her recent paper Annalisa Letizia (Left) and Andreu Casali (centre). |
As we approach International Women’s Day, we spoke to our first woman Researcher of the Month (RotM) at Coffee Table Science, Dr. Marta Llimargas Casanova. Dr. Marta is the Principal Researcher at the Institut de Biologia Molecular de Barcelona where her team studies formation of tissues and organs during development. Her recent publication in PLoS Genetics sheds more light on chitin deposition.
Here’s Dr. Marta speaking more about her publication, women in science and working as a scientist.
CTS: How has your recent publication added to existing knowledge about the chitin deposition?
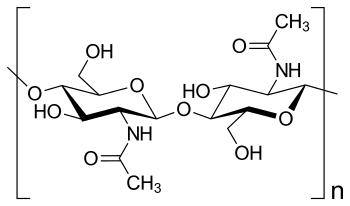
| Chemical structure of chitin (Photo credit: Wikipedia) |
MLC: Chitin is a natural polysaccharide made of UDP-N-acetylglucosamine monomers (a derivative of glucose). It is the second most abundant polymer in nature after cellulose, and from a functional point of view it has activities comparable to those of the protein keratin. Chitin is present in the cell wall of fungi and it is the major component of the exoskeleton of arthropods, providing these animals with structural support and protection against dehydration, infections and predators.
Previous to our work, it was already known that the synthesis of this polymer depends on an enzyme with chitin synthase activity, the so-called Chitin Synthases, CHS, which polymerise the sugar monomers into linear chitin polymers. Actually, there are hundreds of excellent papers describing the activity, regulation, diversity, phylogeny, structure, and biochemical properties of many different chitin synthases. Nevertheless, in spite of so many studies, the exact molecular mechanism by which CHS synthesize chitin remains obscure.
| English: Drosophila sp fly. (Photo credit: Wikipedia) |
In our lab, working with the fruitfly Drosophila melanogaster (a widely used genetic model system), we have discovered a novel activity that together with chitin synthases are also required to synthesize chitin. Our results are important because they provide further insight into the mechanisms controlling chitin deposition and the mechanisms that regulate this deposition during development, providing also a starting point to better understand the molecular mechanisms of production of this polymer.
To make the story short, we have identified an activity (encoded by two interchangeable genes in Drosophila but, interestingly, not in all insects) that, when missing, prevents the deposition of chitin. In normal conditions, chitin is found mainly in two different structures in the Drosophila embryo: in the cuticle, and in the tracheal filament. The cuticle (that forms the exoskeleton) is a layer deposited by the end of embryogenesis that covers and protects the epidermis and the tracheal system. The tracheal system is the respiratory organ of the fly and consists of a network of interconnected branches that reach all tissues and connects to the exterior to allow respiration (comparable to our lungs). In addition to the protective cuticle, the tracheal system also contains a chitin filament that is deposited transiently inside the lumen and that was previously shown to regulate the shape and size of tracheal tubes.
In the absence of the newly identified activity (encoded by two genes named Expansion, Exp, and Rebuf, Reb), none of these structures, the chitin filament and the cuticle form. This leads to embryonic defects (in respiration and morphology) which are not compatible with life, and so the embryos do not reach larval stages. Of note, these same type of defects were also reported for embryos mutant for the main CHS in Drosophila encoded by the gene Kkv. But the most striking result we obtained was that when we express together Kkv and Exp or Reb in cells that normally do not produce chitin, such as other embryonic or adult tissues, they can promote the synthesis of chitin in these tissues. This result is very important, because it identifies the minimal genetic program (established by the two activities Kkv and Exp/Reb) responsible for chitin deposition. None of the two activities alone can trigger chitin deposition, indicating that the enzyme polymerising chitin (Kkv) needs to be regulated. By analysing when and where each of these activities are expressed during embryonic development we have been able to understand and correlate the deposition of chitin during normal development. Finally, and very importantly, we have also observed that when this genetic program is unregulated, it leads to unregulated (advanced, ectopic, or increased) deposition of chitin, and this also prevents normal embryonic morphogenesis. This clearly shows that chitin deposition needs to be highly regulated to allow development to progress.
CTS: How is triggering genes to deposit chitin in places it does not usually exist important? What advantages does it have for us?
MLC: Chitin is found in crustaceans and insects and also in fungi, but not in vertebrates. This makes it an ideal target for the control of insect pests and fungal diseases. Targeting specifically chitin deposition will directly affect insect or fungi survival. Very importantly, pesticides or antifungals designed against chitin deposition are potentially ecologically sensitive because they wouldn’t damage the environment and are non-toxic for human health.
But besides this important application of chitin as a target for antifungals and insecticides, chitin and chitosan (its deacetylated form), have emerged as a new class of natural materials with a wide variety of applications. For instance, they have diverse applications in the biomedical field, such as wound and burn treatment (clotting blood), as a carrier for drug delivery, as a hemostatic for orthopedic treatments, in micro surgery, neurosurgery, in tissue engineering, as a treatment of chronic wounds, ulcers and bleeding (chitin powder). They also have industrial uses, as a degradable and non-toxic biomaterial to manufacture objects, in water treatment, in cosmetics and in textile industry. In summary, their versatility in industry and biomedicine combined with their biocompatibility, biodegradability and low-toxicity lead these biopolymers to the top in R&D efforts. Hence, unravelling the mechanism of chitin polymerization is critical to a gain a better understanding and application of these properties.
CTS: You have mentioned that unregulated chitin deposition has detrimental effects. Could you please elaborate on this?
MLC: Chitin is synthesized by specific tissues at particular stages of development. We have found that this is achieved through regulated expression ( regulated presence) in time and space of the two activities (Kkv and Exp/Reb) required to produce the polymer. This chitin is absolutely required for normal development. Absence of the chitin filament leads to abnormally shaped tracheal tubes that at the end of embryogenesis cannot be filled with air. Tracheal air filling occurs at the end of the embryonic stages (during embryonic development the embryos just breathe by diffusion of gases in the body) when the late embryo and then the larvae require the sufficient gas exchange to cope with their increased physiological activity. Similarly, the chitin deposited in the cuticle is important to provide structural support when the embryos hatch and to shape the larvae. In the absence of this chitin the embryos are flaccid with elastic cuticles and do not hatch. Thus, chitin is an absolute requirement for viability and proper physiological activity.
What it was not known and we have now shown, is that not only the absence of chitin is deleterious, but also its abnormal deposition. We have observed that it interferes with normal organ and tissue formation when it is deposited at earlier stages, or in tissues that do not normally deposit it. When we induce early chitin deposition in the tracheal system, this blocks the rearrangement of cells that occur during morphogenesis. It is important to point out that the tracheal system forms a tridimensional tree of branches inside the embryo during embryonic development out of an original bidimensional flat epithelia. The process of tube formation (the so called tubulogenesis, which also occurs during the formation of other branches tubular structures, like lungs, kidneys, mammary glands or vascular system) involves among other events, several cell rearrangements within the organ to give rise to a tubular network. We find that early and excess chitin deposition prevents these cell rearrangements and hence the branches cannot fully extend and mature. Another example is when we force chitin deposition in the salivary glands, a secretory organ. The salivary glands also arise from a patch of cells in the ectoderm that invaginate and end up forming a tubular structure. But in contrast to the trachea, salivary glands do not normally deposit chitin. When chitin in deposited in the salivary glands the invagination and extension of the tube is seriously compromised, and the glands become extremely malformed and bloated. A final example is when we trigger high and early chitin deposition in the wings. While the cells of the wings normally secrete a chitin based cuticle, its abnormal deposition completely collapse wing formation during morphogenesis, and adults eclose from the pupal case with malformed, unextended and rudimentary wings. Altogether, these results clearly show the need to regulate in time and space the deposition of the required quantity of chitin that allows proper morphogenesis.
CTS: How is premature acquisition of mature traits detrimental to the organism?
MLC: Development requires the precise coordination in time and space of different morphogenetic events. And the order of this series of events needs to be respected to generate normal organisms. For instance, let’s think about the vertebrate lung. During its formation the different conducts (e.g. trachea, bronchia, bronchioli) form in a consecutive manner. Only at the end of the ramification the alveoli form. If the alveolar phase anticipates, it may lead to the formation of a definitive lung before the full ramification had occurred, generating lungs without sufficient exchange surface or not reaching the target tissue. Another example could be tissues in which cell differentiation occurs after a process of cell proliferation. If at the stage of proliferation the cells abnormally acquire its definitive final differentiated state, these are not able to proliferate anymore, generating a rudimentary or malformed organ or tissue.
 |
| Image credit: Dr. Marta Llimargas |
The acquisition of a mature trait usually occurs at the final stage of differentiation of cells, and usually these mature traits constrain the cellular capabilities (in other words, cells become more specialised in their roles but less flexible or potent). In the particular case of chitin that we have analysed, we find that when chitin deposition is anticipated in the trachea the cells become more static, unable to undergo cell rearrangements. It is as if the deposition of such a thick and tough extracellular matrix “freezes” the ability of cells to move, exchange positions, and change their shapes, which is exactly what happens at the end of embryogenesis, when the cells have already formed the structure and need to perform the respiration activity.
CTS:The chitin synthase gene has a complex name (kkv). Is there any story behind its complex naming?
MLC: kkv is the abbreviation of krotzkopf verkehrt, which means something like “snot head, inversed” in german. This mutation was isolated in a genetic screen performed by G Jurgens, E Wieschaus, C Nusslein-Volhard and C Kluding, H in 1984. This work was tremendously important because it represented the basis of our current understanding of the genetic control of embryonic development. One of the mutations isolated in that work was kkv, and the mutation was named with this name by Gerd Jürgens because the late embryos showed head defects and were occasionally inverted inside the eggshell.
CTS: What are your views about women working in science? How has been your experience?
MLC: Personally, I have never felt that I have been judged differently (either positively or negatively) for being a woman, and I would say that in general this type of discrimination is not very common in the scientific world. But it is absolutely true that at top research jobs there are more men than women. This may be somehow surprising if we consider that, for instance in the biomedical field, there are similar numbers of female and male students. Thus, somehow, at some point, there is a clear drop in the proportion of women “fighting for” and getting top positions. The reasons for this fall out of women from science may be diverse, but I would say that a combination of two of them are the main ones: selection of scientists based on masculine criteria, and the personal choice of many women to prioritise other aspects of their lives (or at least not prioritise the professional career).
Dr. Peter A Lawerence, who was my supervisor in Cambridge when I did my postdoctoral stay, elaborated on this subject a few years ago in an opinion paper in PLoS. I quite agree with several of the things he says. There he discusses the fact that the tests and criteria to select top scientists or group leaders generally favour masculine traits, like aggression or self-confidence, and disregard more feminine characteristics like being more gentle, reflective and creative. Equally important is the fact that the scientific career is usually very demanding, and requires a lot of effort and probably to give up other aspects of one’s life (like family, other interests). But these are not the only reasons and there are many more issues to be considered. In summary, gender bias also applies in the scientific world, as a reflection of the society, and it is a major issue that deserves deep analysis. It is important to try to understand the reasons, and try to find out the right measures to close the gender gap (as several scientific institutions in Europe and worldwide do).
CTS: On a lighter note, how difficult is it to explain to your family and friends what your day job is like?
MLC: I would say that in general it is rather easy to explain what we do in the lab. In my experience, I find that people are quite interested in the scientific world, and they are very curious in understanding what we do. Many people feel attracted by a job that they consider very creative, inspiring and altruistic. Hence, to explain in plain words and in a more or less superficial manner the “what we do” is not difficult and there is a wide audience interested in listening. Obviously, more detailed explanations about the “how we do it” are more complex, because they require a background that sometimes is very specialised. But what I find more difficult to explain is the “why we do what we do”, that is, the reason why we investigate a particular subject. This question is very easy to be answered successfully for those working on applied science disciplines, that apply the knowledge to solve practical problems (e.g. diseases, developing new products).
But it is not easy for those of us doing basic research, which has no specific direct application as a goal. I have found myself many times trying to explain why we investigate the development of the fruitfly, and having to justify that the knowledge we acquire provides insight into many different mechanisms and phenomena that will be used by others for more “applied” purposes. Basic research has been and still is, to my opinion, the breadbasket of knowledge that feeds human development. However, it is not always easy to explain to the society the benefits of investing money in basic research, and unfortunately, in recent years, the funding agencies are becoming also difficult to convince.
References
Moussian B, Letizia A, Martínez-Corrales G, Rotstein B, Casali A, & Llimargas M (2015). Deciphering the genetic programme triggering timely and spatially-regulated chitin deposition. PLoS genetics, 11 (1) PMID: 25617778
EDITORIAL (2013). Science for all. Nature, 495 (7439) PMID: 23472264
Lawrence, P. (2006). Men, Women, and Ghosts in Science PLoS Biology, 4 (1) DOI: 10.1371/journal.pbio.0040019