 Last week, the U.S. Food and Drug Administration approved Lenmeldy (atidarsagene autotemcel), the first-ever treatment for children with metachromatic leukodystrophy (MLD). MLD is a very rare but serious disease that attacks the central nervous system. It shuts down body systems that allow people and children to function normally on a day-to-day basis.
Last week, the U.S. Food and Drug Administration approved Lenmeldy (atidarsagene autotemcel), the first-ever treatment for children with metachromatic leukodystrophy (MLD). MLD is a very rare but serious disease that attacks the central nervous system. It shuts down body systems that allow people and children to function normally on a day-to-day basis.
Everyone who develops symptoms of MLD does not survive beyond a decade or two with the disease. Infants who develop symptoms are not expected to survive childhood.
Up until now, the best offering to those with MLD were coping strategies to manage life with the disease. Lenmeldy, manufactured by Orchard Therapeutics, offers a glimmer of hope for a cure.
The catch? It costs $4.25 million.
To learn more about MLD, what Lenmeldy does to treat the disease, and why it’s the world’s most expensive medicine, read on.

MLD can cause paralysis in children
Image Source: Pixabay
What is Metachromiatic Leukodystrophy (MLD)?
MLD is a genetic disease that stops the body from making arylsulfatase A (ARSA), an enzyme that helps break down lipid cells called sulfatides. Without the ARSA enzyme, sulfatides build up in the white matter that protects brain and spinal cord nerves.
In large quantities, the sulfatides are toxic to the cells that produce the white matter, causing it to break down. This leaves the nerves exposed, and eventually, they stop working altogether.
The result is a host of debilitating and devastating symptoms, which tend to get worse as time goes on. People with MLD can lose their cognitive and motor skills, including their ability to think, see, hear, feel, walk, or move at all. They need 24/7 care and expensive equipment for tube-feeding, moving, and using the restroom. In the most extreme cases, life support.
It is unlikely for anyone who has MLD and develops symptoms to survive beyond a couple of decades. Generally, the younger a person starts to exhibit symptoms, the swifter their symptoms progress, and the shorter their expected lifespan. Infants with MLD don’t usually live for more than five or six years with MLD. While MLD is rare (it occurs in only about 0.0025% of the world’s live births), it most commonly afflicts infants and younger children.
What is Lenmeldy?
Lenmeldy is a single-dose gene therapy that uses the patient’s own stem cells to override the disease. The procedure for the treatment can be summed up in four steps:
Step 1: Stem cell mobilization — Doctors must first treat the patient with what’s known as hematopoietic stem cell mobilization. This ejects stem cells from the patient’s bone marrow and releases them into the bloodstream. Doctors can then draw blood from the patient and harvest the stem cells.
Step 2: Genetic Modification — Scientists genetically modify the harvested stem cells. They replace the defunct gene responsible for producing the ARSA enzyme with a functional one. The stem cells get mixed back into the patient’s blood before infusion.
Step 3: Chemotherapy — The patient undergoes a round of high-dose chemotherapy to remove cells from the bone marrow. This opens up sites that the genetically-modified stem cells can graft onto.
Step 4: Lenmeldy Infusion — The stem cells are infused into the patient. They graft back onto the bone marrow and supply the new, fixed DNA code to make the ARSA enzyme to white blood cells throughout the body.
Diagram of gene therapy. Gene therapy works by inserting corrected genes back into stem cells.
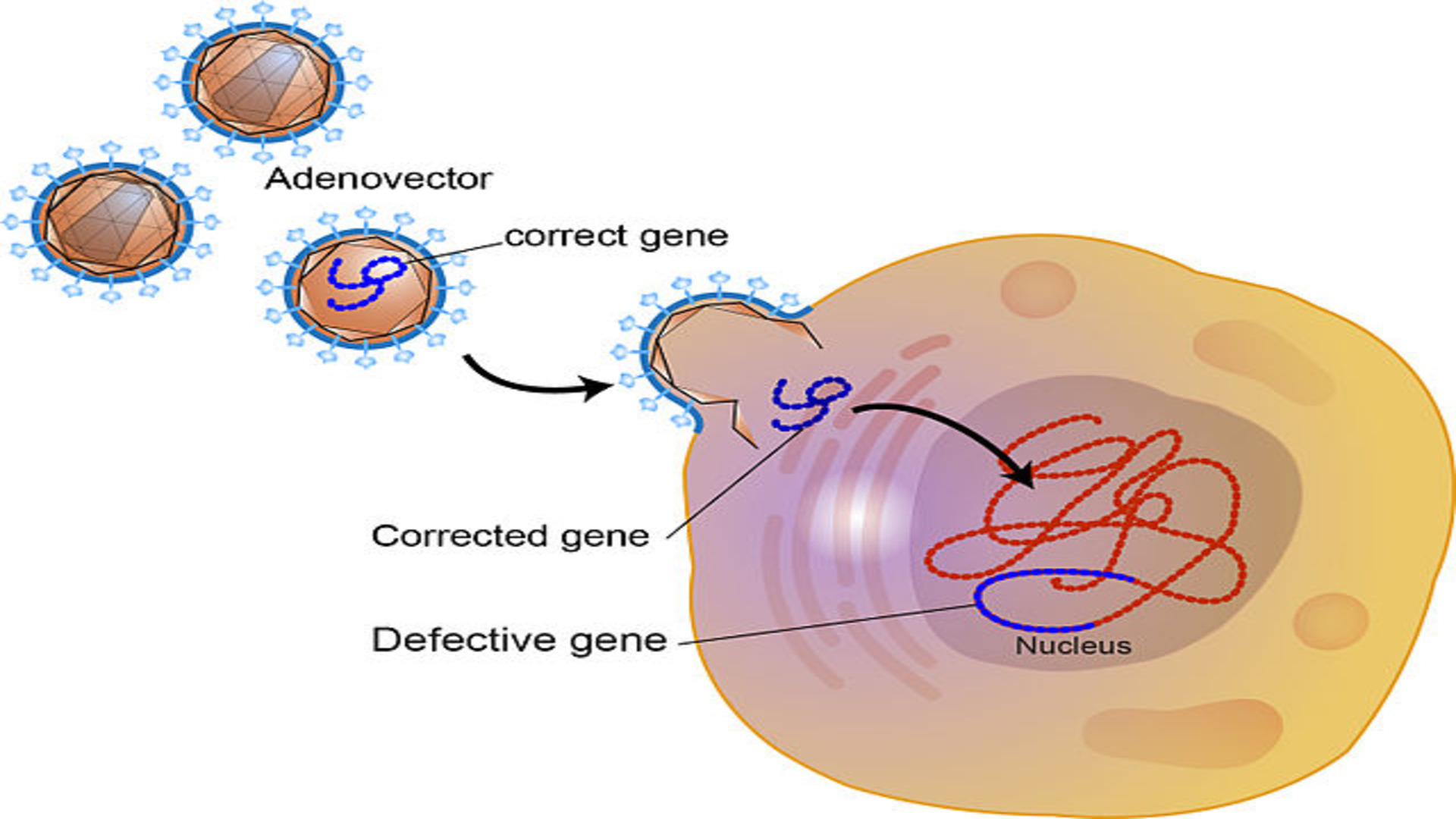
Gene therapy works by inserting corrected genes back into stem cells.
Image Source: Wikimedia Commons
Who is Lenmeldy for
Lenmeldy is only approved for infants and children under 7 who have MLD but either haven’t started showing signs of the disease or have only just started to see symptoms.
Clinical trials on 37 children with MLD treated with Lenmeldy were very promising. Aside from some minor side effects, the treatment prevented the children from developing symptoms.
Symptoms that did crop up progressed less rapidly than in children with MLD who were studied in the past. Most importantly, all the children treated with Lenmeldy survived throughout the duration of the study.
This is good news for people and families of those afflicted by MLD, but keep in mind that it comes at a very, very big cost.
Why is Lenmeldy $4.25 Million?
Many factors went into the price of Lenmeldy beyond just the cost of the treatments and genetic modifications (which are by no means cheap). Like many pharmaceutical companies, Orchard Therapeutics consulted the Institute for Clinical and Economic Review (ICER). This third-party non-profit organization assesses fair medicine prices based on the impact individual that medicine has on the patient.
Because Lenmeldy offers many more years of healthy life to a child who wouldn’t otherwise have them, the ICER can value it at close to $4 million. And if the market for such a treatment is on the order of ~100 people per year, the company likely needs to cash in on each recipient to stay afloat.
This raises some ethical questions about who can afford the treatment and what it means for the research and development of genetic therapies. One question is ringing in many people’s heads after hearing news of this expensive treatment:
How much would you pay to save your child’s life?
If you enjoyed reading our articles, please support us by buying our geeky merchandise on Instagram. Alternatively, you could buy us a coffee or follow us on Facebook, Twitter, Pinterest, or Medium.


